DOI: 10.1055/s-0041-1725054 - volume 43 - Abril 2021
Silva, Arlley Cleverson Belo da; Passos, Jurandir Piassi; Signorini, Roney Cesar; Braga, Antonio; Mattar, Rosiane; Sun, Sue Yazaki
Complete hydatidiform mole (CHM) is a rare type of pregnancy, in which 15 to 20% of the cases may develop into gestational trophoblastic neoplasia (GTN). The diagnostic of GTN must be done as early as possible through weekly surveillance of serum hCG after uterine evacuation.We report the case of 23-year-old primigravida, with CHM but without surveillance of hCG after uterine evacuation. Two months later, the patient presented to the emergency with vaginal bleeding and was referred to the Centro de Doenças Trofoblásticas do Hospital São Paulo. She was diagnosed with high risk GTN stage/score III:7 as per The International Federation of Gynecology and Obstetrics/World Health Organization (FIGO/WHO). The sonographic examination revealed enlarged uterus with a heterogeneous mass constituted of multiple large vessels invading and causing disarrangement of the myometrium. The patient evolved with progressive worsening of vaginal bleeding after chemotherapy with etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine (EMA-CO) regimen. She underwent blood transfusion and embolization of uterine arteries due to severe vaginal hemorrhage episodes, with complete control of bleeding. The hCG reached a negative value after the third cycle, and there was a complete regression of the anomalous vascularization of the uterus as well as full recovery of the uterine anatomy. The treatment in a reference center was essential for the appropriate management, especially regarding the uterine arteries embolization trough percutaneous femoral
Gestational trophoblastic disease (GTD) is a disorder of pregnancy caused by defective differentiation of the trophoblast with both benign and malignant spectrum. As premalignant forms, there are the complete (CHM) and partial hydatidiform mole (PHM). The malignant forms are known as gestational trophoblastic neoplasia (GTN) and classified into invasive mole, choriocarcinoma, placental trophoblastic tumor and epithelioid trophoblastic tumor.1 Diagnosis of GTN is performed based on the criteria of the International Federation of Gynecology and Obstetrics (FIGO) published first in 20022 and updated in 2018.3 The updated criteria consist in rising or stabilization of the serum level of βHCG over at least a period of two or three weeks, respectively, or the histologic diagnosis of choriocarcinoma.3
Gestational trophoblastic disease among women with reproductive desire is treated with chemotherapy, with high chance of cure even in advanced stages.3 However, embolization of the uterine arteries may represent an alternative approach to avoid hysterectomy due to massive bleeding from uterine arteriovenous malformation owing to myometrial tumoral infiltration.45
The present case report was approved by the Ethics Review Board of Universidade Federal de São Paulo, under the number 4.099.490. The need for informed consent was waived due to unsuccessful attempts to contact the patient by email and phone number registered on the service database and patient record.
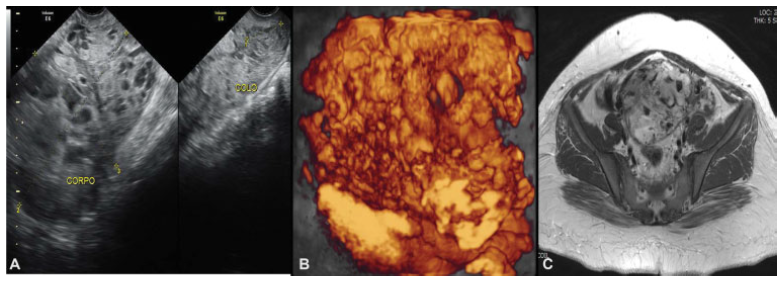
Our patient was a 23-year-old primigravida. She presented to the emergency room with vaginal bleeding at 18 weeks of gestation and, after an investigative transvaginal scan, she was diagnosed with CHM. The patient underwent uterine evacuation by manual intrauterine aspiration. The first βhCG measurement was ˃ 225,000 mlU/ml. Other blood test results showed hemoglobin 9.7 g/dL; hematocrit 30%; TSH 0.003 ml/mL; and free T4 1.69 ng/dL. The result of the histology confirmed the CHM diagnosis. The patient had missed surveillance of hCG for 2 consecutive months after the uterine evacuation. After this period, she went to the emergency room with vaginal bleeding and was subsequently referred to Centro de Doenças Trofoblásticas do Hospital São Paulo, where she was diagnosed with gestational trophoblastic neoplasia (GTN) III:7. The sonographic examination revealed an enlarged uterus measuring 607.8 cm3 with a heterogeneous mass measuring 10.0 × 15.9 × 7.3 cm, constituted of multiple large vessels invading and causing disarrangement of the myometrium (Figs. 1 and 2). Polychemotherapy with etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine (EMA-CO) regimen was immediately prescribed. Since her admission, the patient had had vaginal bleeding that progressively worsened after starting polychemotherapy, probably due to rupture of vessels visualized previously. She received four red blood cells units' transfusion during the second cycle of EMA-CO, and due to the persistence of the severe vaginal hemorrhage, embolization of the uterine arteries was performed successfully as a treatment. Embolization was performed by percutaneous puncture accessing the right femoral artery, and each uterine artery was selectively catheterized with a 5-F glide catheter and embolized with geolfoam particles (Fig. 3). Although the patient had shown liver toxicity after the first cycle of chemotherapy, the treatment could be done without delay after adjusting the doses. The hCG reached a negative value (Table 1) after the third cycle, and there was a complete regression of anomalous vascularization of the uterus as well as recovery of the uterine anatomy seen by pelvic ultrasonography (Fig. 4). The patient was followed monthly until September 2017 and, later, every 3 months up to July 2018 under hCG surveillance and contraception recommendation.
Fig. 1
Uterine images before chemotherapy (June 9th, 2016) demonstrating loss of myometrium stratification due to heterogeneous image, mainly vascular, invading the whole uterus and cervix. A. Pelvic transvaginal ultrasonography B-mode. B. Transvaginal three-dimensional HD-flow multiplanar view scan. C. Pelvic magnetic resonance imaging (MRI)

Fig. 2
Transvaginal scan before the second cycle of polychemotherapy (June 25th, 2016)

Fig. 3
Pelvic angiogram. A. Ongoing embolization. B. Completed embolization (July 6th, 2016)

Fig. 4
Transvaginal scan after the last cycle of polychemotherapy (December 14th, 2016)

Table 1
Clinical and laboratorial data
| Date | Event | hCG mUI/mL | Others |
|---|---|---|---|
| April 14th, 2016 | Molar suction uterine evacuation | > 22,5000 | Hb 9.7 g/dL, Htc 30% |
| June 9th, 2016 | Referred to CDTHSP | 229,965 | |
| June 10th and 11th, 2016 | EMA | ||
| June 17th, 2016 | CO | ||
| June 28th and 29th, 2016 | EMA | 24,633 | Hb = 10.1 g/dL; Htc 29.3% |
| July 5th, 2016 | CO | Hb = 6.5 g/dL, Htc = 20.3% Transfusion 4 RBC SBP = 90 × 60 mm Hg, CF = 120 bpm |
|
| July 6th, 2016 | Embolization of the uterine arteries | − | |
| July 12th and 13th, 2016 | EMA | 1,137 | |
| July 25th 2016 | CO | ||
| August 2nd and 3rd, 2016 | EMA | 4.5 | |
| August 9th, 2016 | CO | ||
| August 16th and 17th, 2016 | EMA | 3 | |
| August 23rd, 2016 | CO | ||
| August 30th and 31st, 2016 | EMA | 1.3 | |
| September 6th, 2016 | CO |
Abbreviations: CDTHSP, Centro de Doenças Trofoblásticas do Hospital São Paulo; CF, cardiac frequency; EMA-CO chemotherapy regimen: etoposide, methotrexate, actinomycin D, cyclophosfamide, vincristine; Hb, hemoglobin; hCG, human chorionic gonadotropin; htc, hematocrit; RBC, red blood cells; SBP, systolic blood pressure.
Vaginal bleeding has been reported as the most common symptom in the cases of hydatidiform mole. However, the symptoms and signs that have been associated with molar pregnancy are getting less common in the practice due to the greater availability of ultrasound scans and hCG tests in the first trimester of pregnancy, as a routine or to evaluate vaginal bleeding, affording the earlier diagnosis of molar pregnancy.67 In the present case report, the patient sought the emergency room due to vaginal bleeding, but without other symptoms such as hyperemesis, respiratory discomfort, and hyperthyroidism, which could indicate a more advanced disease.
In view of the initial hydatidiform mole diagnosis, the management was accomplished by performing a uterine evacuation in the operating room, as recommended. We chose the suction evacuation technique of manual vacuum aspiration, which has been associated with lower risk of uterine synechia, compared with electric vacuum aspiration.8
The diagnosis of GTN is based on surveillance of serum levels of hCG, and this malignant process is suggested by the presence of plateau or rising of hCG serum levels on two or three consecutive weekly samples.39 Our patient had not performed the recommended surveillance of serum hCG levels after the uterine evacuation; then, 2 months later, she evolved with an elevation of hCG serum levels associated with vaginal bleeding. These features along with the pelvic ultrasonography demonstrating lesions on the uterine wall allowed the diagnosis of GTN.
Once the diagnosis of GTN has been made, the anatomical involvement of the disease and risk should be defined based on the FIGO criteria (Charts 123), and the patient must be classified as low or high risk. Patients with a score of 0 to 6 are defined as low risk because they are likely to respond to single-drug therapy, and those with a score higher than 6 are considered as high risk of resistance to single-drug chemotherapy. According to this classification, the multiple drugs chemotherapeutic regimen is preferred for the high-risk patients.13
Chart 1
Gestational trophoblastic neoplasia anatomic staging (FIGO/WHO)
| Stage I | Disease confined to the uterus |
|---|---|
| Stage II | GTN extends outside of the uterus but limited to the genital structures (adnexa, vagina, broad ligament) |
| Stage III | GTN extends to the lungs, with or without know genital tract involvement |
| Stage IV | All other metastatic sites |
Abbreviations: FIGO/WHO, The International Federation of Gynecology and Obstetrics/World Health Organization; GTN, gestational trophoblastic neoplasia.
Chart 2
Gestational trophoblastic neoplasia prognostic scoring system (FIGO/WHO)
| Scores | 0 | 1 | 2 | 4 |
|---|---|---|---|---|
| Age | < 40 | ≥ 40 | − | − |
| Antecedent pregnancy | Mole | Abortion | Term | − |
| Interval months from index pregnancy | < 4 | 4–7 | 7–13 | ≥ 13 |
| Pretreatment serum hCG (IU/Ml) | < 103 | 103–104 | 104–105 | ≥ 105 |
| Largest tumor size (including uterus) | − | 3–5 cm | ≥ 5 cm | − |
| Site of metastasis | Lung | Spleen, kidney | Gastrointestinal | Liver, brain |
| Number of metastasis | − | 1–4 | 5–8 | > 8 |
| Previous failed chemotherapy | − | − | Single drug | 2 or more drugs |
Abbreviations: FIGO/WHO, The International Federation of Gynecology and Obstetrics/World Health Organization; hCG, human chorionic gonadotropin.
After the confirmed diagnosis of GTN, a further investigation is required, and taking into consideration that pulmonary metastases are the most common ones, a chest radiograph is crucial. If lesions are noted on chest X-ray, magnetic resonance imaging (MRI) of the brain and abdominal computed tomography (CT) are indicated to exclude metastatic disease in other sites, such as the liver.236 In the present case, the patient was classified as high risk and had pulmonary metastasis identified. In view of the findings, a multiple agent therapy was indicated, and the regimen with EMA-CO was chosen for being considered in the literature the first-line therapy for high-risk cases.136 It is essential to highlight the importance of rigorous postmolar follow-up for early detection of GTN, which would enable less aggressive regimens of treatment, such as in this case.10
The toxicity of the EMA-CO regimen is usually well tolerated, being more common the hematological; however, there are also reports of effects on the gastrointestinal tract and peritoneal and pleural serositis.11 The patient in the current case showed toxicity in the gastrointestinal tract with the use of the EMA-CO chemotherapy regimen, which could be managed through an adjustment of the doses.
The large vessels of the arteriovenous malformations invading the myometrium in communication with the endometrial cavity, almost reaching the serosa, represented a threat of vaginal bleeding and uterine perforation. Embolization of uterine arteries was an alternative approach to control the vaginal bleeding and avoid hysterectomy in this nulliparous patient.
Studies in the literature support a high pregnancy rate despite prior chemotherapy with no increased adverse outcomes.1912 Furthermore, as previously demonstrated, women of childbearing age were able to get pregnant after uterine arteries embolization,131415 this management was adopted when the patient was found having severe hemorrhage episodes over the chemotherapy.
In addition, a previous study showed that patients who underwent uterine artery embolization to treat hemorrhage from arteriovenous malformation due GTN had favorable outcomes with low rate of complications and recovered well after the procedure, with normal uterine function and regular menstrual cycles.16
Owing to the complexity of the disease and its severity, patients must be treated and followed-up in a specialized center with structure and experience in the management of gestational trophoblastic disease, therefore increasing survival as well as the chances of cure of patients with this disease.4
In the case presently reported, the fact that the patient was referred to a tertiary and specialized service in GTD was extremely important for the successful outcome. In these reference centers, a multidisciplinary team is available to offer the best treatment to cure the patient and maintain her reproductive function. Selective embolization of uterine arteries is one of the most effective techniques to avoid hysterectomy due to intractable pelvic hemorrhage in cases of GTN in nulliparous women.
Brown J, Naumann RW, Seckl MJ, Schink J. 15years of progress in gestational trophoblastic disease: Scoring, standardization, and salvage. Gynecol Oncol. 2017;144(01):200-207. Doi: 10.1016/j. ygyno.2016.08.330
FIGO Oncology Committee. FIGO staging for gestational trophoblastic neoplasia 2000. Int J Gynaecol Obstet. 2002;77(03): 285-287. Doi: 10.1016/s0020-7292(02)00063-2
NganHYS, SecklMJ, Berkowitz RS, et al.Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet. 2018;143(Suppl 2):79-85. Doi: 10.1002/ijgo.12615
Braga A, Lima L, Parente RCM, et al. Management of symptomatic uterine arteriovenous malformations after gestational trophoblastic disease. The Brazilian experience and possible role for depot medroxyprogesterone acetate and tranexamic acid treatment. J Reprod Med. 2018;63(5-6):228-239
Lim AKP, Agarwal R, Seckl MJ, Newlands ES, Barrett NK, Mitchell AWM. Embolization of bleeding residual uterine vascularmalformations in patients with treated gestational trophoblastic tumors. Radiology. 2002;222(03):640-644.Doi:10.1148/radiol.2223010035
Seckl MJ, Sebire NJ, Fisher RA, Golfier F, Massuger L, Sessa CESMO Guidelines Working Group. Gestational trophoblastic disease: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi39-vi50. Doi: 10.1093/annonc/mdt345
Sun SY, Melamed A, Joseph NT, et al. Clinical presentation of complete hydatidiform mole and partial hydatidiform mole at a regional trophoblastic disease center in the United States over the past 2 decades. Int J Gynecol Cancer. 2016;26(02):367-370. Doi: 10.1097/IGC.0000000000000608
Padrón L, Rezende Filho J, Amim Junior J, et al. Manual compared with electric vacuum aspiration for treatment of molar pregnancy. Obstet Gynecol. 2018;131(04):652-659. Doi: 10.1097/AOG.0000000000002522
Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010;376(9742):717-729. Doi: 10.1016/S0140-6736(10)60280-2
Quinn M, Murray J, Friedlander M, et al. EMACO in high risk gestational trophoblast disease-the Australian experience. Gestational Trophoblast Subcommittee, Clinical Oncological Society of Australia. Aust N Z J Obstet Gynaecol. 1994;34(01):90-92. Doi: 10.1111/j.1479-828x.1994.tb01047.x
Tranoulis A, Georgiou D, Sayasneh A, Tidy J. Gestational trophoblastic neoplasia: a meta-analysis evaluating reproductive and obstetrical outcomes after administration of chemotherapy. Int J Gynecol Cancer. 2019;29(06):1021-1031. Doi: 10.1136/ijgc-2019-000604
Torre A, Fauconnier A, Kahn V, Limot O, Bussierres L, Pelage JP. Fertility after uterine artery embolization for symptomatic multiple fibroidswith no other infertility factors. Eur Radiol. 2017;27 (07):2850-2859. Doi: 10.1007/s00330-016-4681-z
Touhami O, Gregoire J, Noel P, Trinh XB, Plante M. Uterine arteriovenous malformations following gestational trophoblastic neoplasia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2014;181:54-59. Doi: 10.1016/j.ejogrb.2014.07.023
Braga A,Mora P, de Melo AC, et al. Challenges in the diagnosis and treatment of gestational trophoblastic neoplasia worldwide. World J Clin Oncol. 2019;10(02):28-37. Doi: 10.5306/wjco.v10. i2.28
Belfort P, Braga A, Freire NS. [Uterine arteriovenous malformation after gestational trophoblastic disease]. Rev Bras Ginecol Obstet. 2006;28(02):112-121. Doi: 10.1590/S0100-72032006000200007 Portuguese.